Blueprint Medicines was founded on the belief that the research and development of innovative medicines could be accelerated. To bring this idea to life, we have used our proprietary scientific platform to develop potent and selective inhibitors for highly sought-after targets, and we seek to make a difference for patients with high medical needs.
Leveraging our foundational research capabilities, we recognized early on that targeting the KIT receptor, including the KIT D816V mutation, could be an approach for developing novel therapies that treat the root causes of certain diseases. Since then, we have developed leading expertise in understanding the role of KIT and mast cell biology, including in allergic/inflammatory diseases like systemic mastocytosis (SM).1
SM is a rare mast cell disorder that can lead to debilitating and burdensome symptoms with a profound impact on patients’ lives, and the disease is characterized by the uncontrolled proliferation and activation of mast cells.2-4 This abnormal mast cell activity typically causes chronic, severe and unpredictable symptoms across organ systems.3 The KIT D816V mutation drives approximately 95% of all SM cases.5-7 Approximately 1 in every 10,000 people has SM, with a majority of patients living with indolent SM (ISM) and a minority of patients having advanced SM.5,8
What are mast cells?
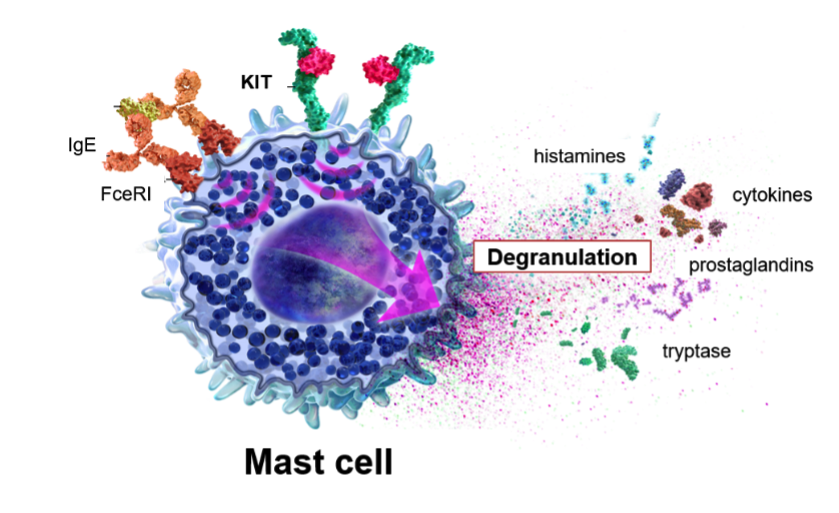
Mast cells are a core part of the immune system, and addressing mast cell biology can help treat a number of allergic/inflammatory diseases. Mast cells are the central effector cells driving many diseases,9 and KIT is clinically validated as a key controller of mast cell activation, proliferation and survival. Upon activation, mast cells release pro-inflammatory molecules, also known as mediators, like histamines, proteases and prostaglandins in a process called degranulation; these mediators cause most of the symptoms associated with allergic/inflammatory diseases.9,10
Our leading expertise in mast cell biology
We’re pioneering an innovative approach to treat mast cell- or KIT-driven diseases by directly inhibiting activation and degranulation at the source, with the goal to impact the lives of more patients in the future. Today, our pipeline consists of an approved medicine and multiple investigational programs that demonstrate our proven track record of leadership in mast cell diseases.
“Key components of our success include our deep understanding of mast cell biology, and our expertise in designing potent and selective molecules that target root causes of disease,” says Fouad Namouni, M.D., President of Research and Development at Blueprint Medicines. “At Blueprint, we have built what we believe is one of the most advanced mast cell drug discovery capabilities in the industry.”
As we continue to hone this expertise, we reflect on our work to-date to bring our innovation to the mast cell disease community, including through the U.S. approval of our therapy for the treatment of ISM one year ago. “ISM is rare, and I didn’t know much about it when I first joined Blueprint. The ‘I’ stands for indolent, and that is such a misnomer,” says Maria Roche, Vice President of Clinical Development at Blueprint Medicines. “This is a really challenging disease. Many of these patients have significant symptoms despite the efforts of their healthcare providers to try and treat them. I’m glad that we are bringing our medicine to people who really struggle with their disease.”
We remain steadfast in our commitment to delivering important medicines to patients globally, including those who have previously lacked treatment options that target the root causes of their disease. As we continue to deepen our understanding of mast cell biology, we believe we are uniquely positioned to drive scientific leadership, pioneer additional treatment advances and improve outcomes for more people living with allergic/inflammatory diseases.
References
